Transmembrane signaling involves sensing and communicating with the extracellular environment. Part of the transmembrane signaling is a process known as autoproteolysis, which is key in different functions of a cell.
In this process a peptide chain, which is a string of amino acids, is cleaved at a specific site by the peptide itself. This is typical of eukaryotes, though a defined, functional autoproteolytic event for transmembrane signal transduction has not been seen in bacteria until now.
Now, however, this signaling process thought to be almost entirely exclusive to eukaryotes has been confirmed in bacterial species Clostridium thermocellum, according to researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS).
Using the bacteria Clostridium thermocellum, researchers found an autoproteolytic effect that is essential in activating further transmembrane signaling down the line, similar to the widely known autoproteolysis mechanism seen in eukaryotes.
“Not only is this a novel observation, but the presence of a conserved site for the automatic cleavage of the amino acid chain suggests a more unique and complex world of bacterial signaling than many would’ve thought possible,” said Dr. CHEN Chao, first author of the study.
The study was published in Science Advances on July 7.
Autoproteolysis for transmembrane signaling is rarely reported in prokaryotes, and it is usually saved for protease maturation. But in the case of C. thermocellum, a bacterium that feeds off of tough plant material (lignocellulolytic), it appears to be an essential part of signal transduction. The process occurs within the “in-between” spaces of the inner cytoplasmic membrane and bacterial outer membrane called the periplasm, in a conserved amino acid sequence known as asparagine-proline.
This conserved sequence is found on an anti-σ factor “RsgI,” a protein that is responsible for the sensing and transduction of signals to the cells and works to inhibit the activities of σ-factor “SigI,” which starts the transcription of RNA from a DNA template and functions to transcribe specific genes.
Lignocellulolytic bacteria, such as C. thermocellum in the study, have extracellular enzyme complexes called “cellulosomes” which are the targets regulated by multiple pairs of RsgI/SigI factors, and the cellulosome functions to degrade hard plant materials the bacteria consume, like cellulose, hemicellulose and pectin.
“As a unique class of σ/anti-σ factors, the functional mechanism of SigI/RsgI has still not beenfully elucidated,” said Prof. FENG Yingang, corresponding author to the study.
Given the distinctive mechanism of the autoproteolysis seen in C. thermocellum it can be rightfully assumed there is still work to be done to uncover more about the ins and outs of how this autoproteolysis works within lignocellulolytic bacteria, specifically when it comes to the cellulosome and the accompanied regulatory actions performed by anti-σ/σ factors, such as the binding of RNA polymerase to the promoter region to begin transcription.
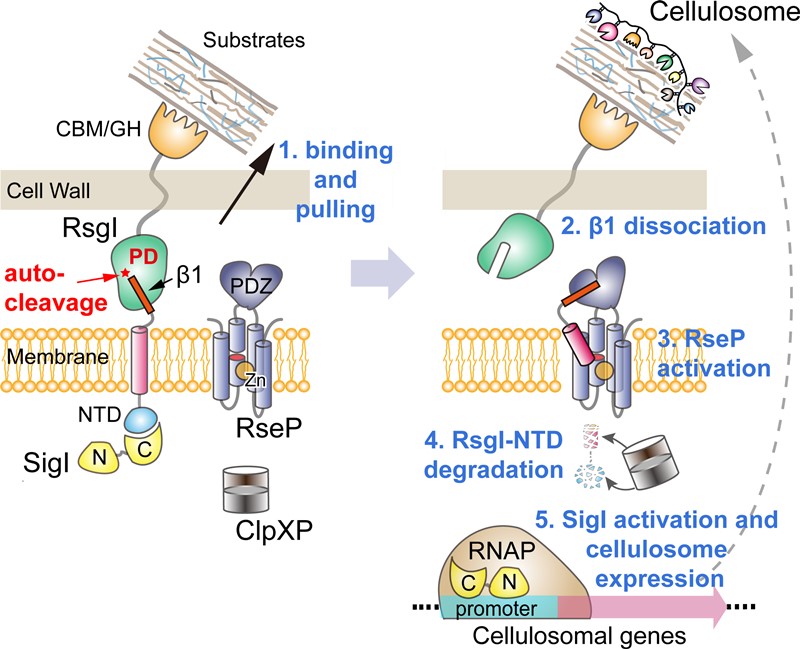
Proposed model of transmembrane signal transduction of RsgI in C. thermocellum (Image By CHEN Chao and FEGN Yingang)
(Text by CHEN Chao, FEGN Yingang)