Tartaric acids have L(+)- and D(-)-type enantiomers, and they are chiral precursors for synthesis of many fine chemicals and pharmaceuticals. In nature, tartaric acid is mainly L(+)-type, while D(-)-tartaric acid is less present in nature. Because tartaric acid is a small molecular weight compound with mirror symmetry, it is also difficult to prepare highly pure enantiomers by chemical method. By using biological catalysts such as epoxide hydrolase which hydrolyzescis-epoxysuccinate, high-purity tartaric acid can be prepared efficiently, wherein the hydrolases producing L(+)- and D(-)-tartaric acid were called CESH[L] and CESH[D], respectively. Although CESH[L] and CESH[D] were found in nature in the 70 's, and subsequently used in the industrialized production of tartaric acid, their stereoselective catalytic mechanism was unclear due to the lack of crystal structure of the two enzymes. In addition, there are many types of epoxide hydrolase in nature, and some studies have shown that it is possible to hydrolyze different kinds of epoxide substrates by molecular engineering of these hydrolases and they can be used to prepare a variety of chiral compounds, which is of great significance in the development of biological catalysts.
Based on the expression and purification of CESH[D] with high purity, the Metabolomics Group in Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences successfully solved the high resolution crystal structure of CESH[D] by cooperating with researchers at Tsinghua University. The mechanism of stereoselective catalysis was elucidated from analysis of the CESH[D] structure. These results were recently published inChem. Commun.(https://doi.org/10.1039/C8CC04398A). The structure of CESH[D] shows that a zinc ion plays a key role in the substrate binding, while the peripheral residues precisely fixe the catalytic residues and substrates by a large number of hydrogen bonds, thus achieving a high-precision stereoselective catalysis to produce highly enantiomerically pure D (-) tartaric acid. The structure and catalytic mechanism of CESH[D] elucidated in this research will also provide the basis for the design and development of novel biological catalysts based on these enzymes.
The above work was supervised by Professor Yingang Feng and Professor Qiu Cui in QIBEBT, and Professor Xinquan Wang in Tsinghua University cooperatively. Associate Professor Sheng Dong in QIBEBT is the first author of the paper. The study has supported by the National Natural Science Foundation of China.
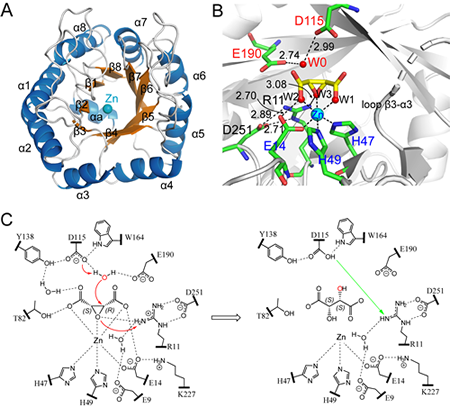
Figure 1. The structure and catalytic mechanism of cis-epoxysuccinate hydrolase CESH[D] which can produce D (-) tartaric acid. (A) The overall structure of CESH[D]. (B) The active site structure of CESH[D] and the proposed substrate-binding mode. (C) Catalytic mechanisms proposed from structural analysis.
Related publications:
Sheng Dong, Xi Liu, Gu-Zhen Cui, Qiu Cui*, Xinquan Wang*, Yingang Feng* (2018) Structural insight into the catalytic mechanism of a cis-epoxysuccinate hydrolase producing enantiomerically pure D(-)-tartaric acid.Chem. Commun.54(61):8482-8485.https://doi.org/10.1039/C8CC04398A
Gu-Zhen Cui, Shan Wang, Yifei Li, Yi-Jun Tian, Yingang Feng, Qiu Cui* (2012) High yield recombinant expression, characterization and homology modeling of two types ofcis-epoxysuccinic acid hydrolases.Protein J.31(5):432-438.http://dx.doi.org/10.1007/s10930-012-9418-5
Shan Wang, Gu-Zhen Cui, Xiang-Fei Song, Yingang Feng*, Qiu Cui* (2012) Efficiency and stability enhancement ofcis-epoxysuccinic acid hydrolase by fusion with a carbohydrate binding module and immobilization onto cellulose.Appl. Biochem. Biotechnol.168(3):708-717.http://dx.doi.org/10.1007/s12010-012-9811-8